Abstract
Introduction: Multiple myeloma (MM) is an incurable plasma cell malignancy characterized by osteolytic bone disease and immunosuppression. Transforming growth factor-beta (TGF-β) supports MM progression through its role in stimulation of IL-6, Th17/regulatory T cell development, hematopoietic suppression, and its promotion of catabolic bone remodeling. Inhibition of the proteasome has emerged as a powerful strategy for treatment of MM. Proteasome inhibitors (PIs) have improved overall survival in patients with MM, but the majority develop drug resistance. Therefore, a novel therapeutic strategy is needed for better treatment of MM. Vactosertib (VACTOSERTIB) is an orally bioavailable small molecule inhibitor of the TGF-β type I receptor kinase (TβRI) currently in a phase I clinical trial (NCT03143985) in combination with the immunomodulatory drug, pomalidomide. In this study, we first investigate if Vactosertib is capable of diminishing myeloma progression either as a single agent or in combination with the PI, ixazomib.
Methods: Expression levels of TGF-β1 and phospho-Smad2/3 was examined in human and mouse MM cells and bone marrow by Western blot and immunohistochemistry (IHC). Cellular and molecular assays were performed in human RPMI8226, ARH-77 and PIs-resistant RPMI8226 as well as murine 5T33MM cells via MTT, apoptosis, real-time PCR and Western blot. For preclinical evaluation, C57BL/KaLwRij mice bearing 5T33MM cells expressing luciferase were treated with Vactosertib, ixazomib, or the combination of Vactosertib plus ixazomib for 3 weeks, and evaluated for MM growth by bioluminescence imaging (BLI). Peripheral blood IL-6 and monoclonal protein concentration, M-spike, were measured by ELISA. Fluorescence-activated cell sorting (FACS) analyses were used for immunological evaluation, such as myeloid derived suppressor cells (MDSCs), Foxp3+ regulatory T cells (Tregs) and NK cells. Femur trabecular bone density and microarchitecture were assessed by 3D micro-CT system.
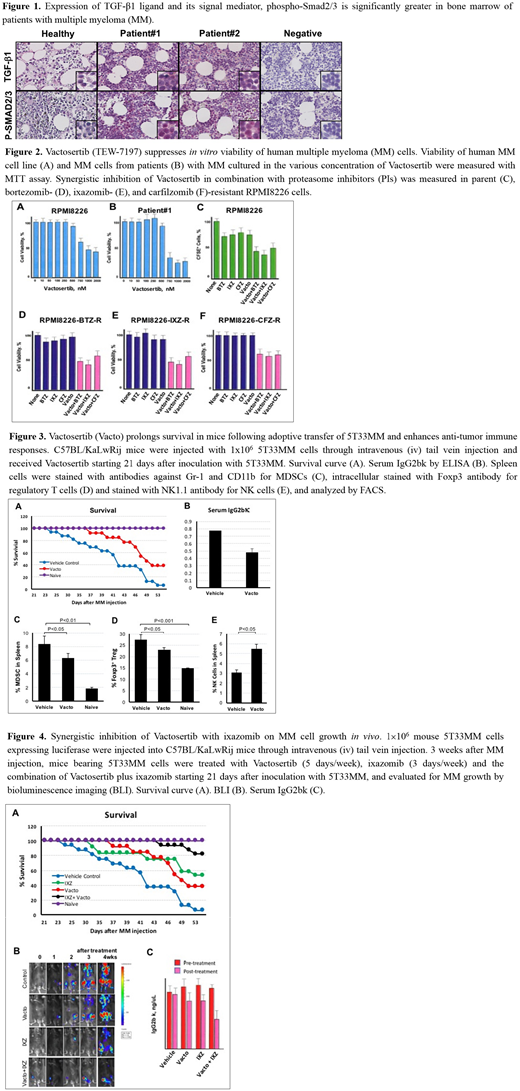
Results: We first evaluated the expression of the TGF-β1 ligand and its receptor activated signaling intermediates, Smad2/3 in bone marrow of patients with MM. There is an accumulation of the type 1 isoform of the TGF-β ligand within the tumor microenvironment (TME) and of phospho-Smad2/3 in myeloma cells within the bone marrow of patients (Figure 1). In vitro studies utilizing the TbRI inhibitor Vactosertib (TEW-7197) with murine 5T33MM, human RPMI8226 (Figure 2A), U266, ARH-77 cell lines, and tumor cells from patients with MM (Figure 2B) cultured demonstrate a dose-dependent reduction in cell viability in cultures of these MM cells continuously exposed to Vactosertib. Synergistic inhibition of Vactosertib in combination with PIs was also observed in parent (Figure 2C), bortezomib- (Figure 2D), ixazomib- (Figure 2E), and carfilzomib-resistant RPMI8226 cells (Figure 2F). We also found that there is an accumulation of the TGF-β1 within the TME and phospho-Smad2/3 within the MM at advanced stages in the bone marrow of C57BL/KaLwRij mice harboring the 5T33MM myeloma cells. Oral administration of Vactosertib as a single agent inhibited MM progression as measured by mortality (Figure 3A), peripheral blood monoclonal protein concentration and BLI (Figure 3B). While the expansion of CD11b+Gr-1+ MDSCs (Fig. 3C) and the population of Tregs (Fig. 3D) in the spleen were reduced, NK cells were increased by Vactosertib alone (Fig. 3E). Combination therapy of Vactosertib plus ixazomib exhibited a synergistic anti-tumor effect when compared to either Vactosertib or ixazomib alone by a decrease in mortality (Figure 4A) and significant reduction in both the BLI and M-spike before and after treatment (Figure 4B, C). Furthermore, Vactosertib single therapy enhanced the bone thickness and reduced trabecular separation in comparison to control mice.
Conclusions: Taken together, our data demonstrate that Vactosertib (a small molecule inhibitor of the TGF-β type I receptor kinase) suppresses viability of human MM cells, and is associated with a potent anti-myeloma effect, suppressing bone resorption and modulating the MM TME in a immunocompetent preclinical model of MM. These data provide a rationale for clinical evaluation of the combination therapy of Vactosertib and ixazomib as a potential therapeutic strategy to improve outcomes in patients with MM.
Kim:MedPacto: Other: Founder/CEO. Malek:Takeda: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal